The pandemic response team instituted COVID-19 policies that are playing a role in the rise of mortality rates over the past three years. Lockdowns, the restriction of treatment, ventilation of Covid-positive patients, and COVID-19 “vaccines” caused hundreds of thousands of lives. Another approved COVID-19 treatment protocol is the drug remdesivir. Let’s examine the safety and effectiveness of remdesivir.
Previous Remdesivir Studies
Remdesivir is not a new drug – Fauci has tried to get it approved for a while. The safety and effectiveness of remdesivir in relation to other viruses has exhibited poor results. It showed to have very low safety and efficacy profiles. Remdesivir showed lethal side effects during clinical trials in 2016 for Zika, in 2018 for Ebola, and in 2020 in China. Many studies had to be discontinued after 5-10 days because of severe adverse events or death associated with this drug.
As a matter of fact, 70%-75% of participants who received remdesivir developed adverse side effects (acute kidney failure, liver damage, septic shock, and hypotension). Additionally, 53% of patients receiving remdesivir died. No wonder why the drug was never approved.
Emergency Use Authorization
Despite failed results, Fauci pushed to give Remdesivir an Emergency Use Authorization (EUA). This is when medical products (vaccines, drugs, testing procedures, etc) are allowed to be marketed and used on the population despite not being FDA-approved. The EUA status allows companies manufacturing these products to be exempt from liability so they can’t be sued as well.
Drugs need to pass certain criteria in order to get the EUA:
- The drug is supposed to be effective in treating or preventing a disease – in this case, COVID-19
- Its known potential benefits are supposed to outweigh its potential risks
- There can’t be other adequate, approved, and available alternatives for diagnosing, preventing, or treating COVID-19.
Taking an unbiased stance, we can’t say that remdesivir falls under that umbrella. Previous studies showed it to be pretty unsafe and non-effective for the viruses aiming to treat. Plus, there are available therapeutics treating COVID-19 successfully. Nevertheless, this drug was granted approval on May 1, 2020.
Remdesivir Studies on COVID-19
Fauci claimed that remdesivir had a clear-cut significant positive effect. The following are the studies funded by the NIAID addressing the safety and effectiveness of remdesivir in relation to COVID-19:
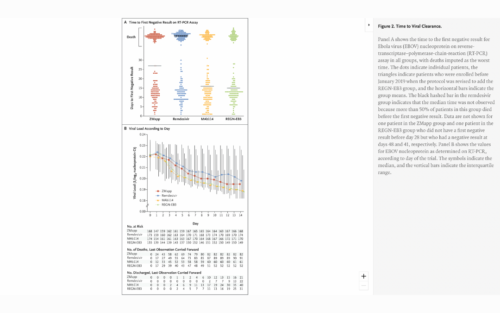
? The ACTT (Adaptive Covid-19 Treatment Trial) study reported that after a five-day course of remdesivir patients improved. By day 15, 7% of the participants given remdesivir died, compared with 11% of the placebo group. However, by day 29, 11% of those receiving remdesivir vs 15% with placebo died. From these results, claiming that this drug is highly effective is fraudulent since there was not a statistically significant difference between the two groups. Remdesivir was not much more beneficial than having no drug at all.
?? 25% of those receiving remdesivir presented serious adverse events (acute respiratory failure, decreased glomerular filtration rate, decreased hemoglobin level, decreased lymphocyte count, anemia, pyrexia, hyperglycemia, increased blood creatinine level, and increased blood glucose level).
?? 12% of patients receiving remdesivir had discontinued treatment or withdrew consent because of adverse events by day 10 and, 3% by day 29. By day 15, 7% of patients receiving remdesivir died, and 12% died by day 29. We don’t know what happened to those who terminated participation or consent.
?? Interestingly enough, researchers changed the variables during the study. They were going to score patients on a scale from 1 (dead) to 8 (not hospitalized, with no restrictions on activities). Yet, they changed the measurement to a three-point scale in the middle of the study. That’s against protocol in science. You are not supposed to do that because you don’t get real results associated with the drug – fraudulent researchers do that to fit results to the narrative they want to portray.
?? Additionally, researchers ended the placebo group and gave everyone remdesivir – just like they did with the COVID-19 “vaccines” to draw conclusions on effectiveness. Eliminating the placebo group limits the capability to collect data and make real comparisons between the experimental and placebo control groups. Even though Fauci called it a placebo-controlled trial, it really wasn’t.
? Fauci used another study analyzing 53 patients. The results showed that 13% of patients receiving remdesivir died and 18% receiving invasive ventilation died versus 5% of those receiving noninvasive oxygen support or drug. The only group showing a significantly positive result was the one with remdesivir or ventilators.
?? Furthermore, 8% of patients discontinued remdesivir treatment prematurely due to adverse events (worsening renal failure, multiple organ failure, elevated aminotransferases, maculopapular rash).
Clinical trials using remdesivir addressing COVID-19 were not much different than previous ones. Remdesivir can’t be classified as effective or safe even though Fauci declared that based on the studies that he sponsored. Nonetheless, the FDA approved remdesivir on October 22, 2020.
? Studies with no conflicts of interest or Fauci’s interference showed different results. The WHO did a study (Solidarity trial) that used a much bigger sample than NIAID’s trials. It followed 11,266 adults in 30 countries and their results do not corroborate Fauci’s claims.
?? Their research concluded that there is NO scientific evidence showing a decrease in mortality, the need for mechanical ventilation, or any clinical improvement in patients receiving remdesivir. Consequently, since 2020, the WHO recommends against using remdesivir in COVID-19 patients. Yet, remdesivir is the approved protocol for hospitalized patients in the US.
Déjà Vu
Despite Remdesivir’s undesirable health record, Fauci pushed it and the FDA approved it. This is part of Fauci’s playbook – he had the same approach during the AIDS epidemic. First, he propagates fear-mongering campaigns about the lethality of a virus in every media channel. Then, he fast-tracks the approval of expensive pharmaceuticals that benefit him financially and make millions for his pharma connections.
During the AIDS epidemic, he pushed AZT despite the fact that it showed to have low safety profiles. He even funded sadistic experiments using children as lab rats with taxpayers’ money. The clinical trials for AZT presented countless flaws with questionable experimental procedures and conclusions. Even though outcomes showed numerous damaging adverse effects, he fast-tracked its approval.
Thought-Provoking Facts Involving Remdesivir
Remdesivir’s adverse events are so widespread that lawsuits are taking place. Attorneys Daniel Watkins and Hamilton (Declare Truth) are representing several families who are suing hospitals for the death of patients after receiving remdesivir without obtaining informed consent. Plus, Attorney Tom Renz’s research shows that about 25.9% of COVID-positive patients who received remdesivir died over the past few years.
Conflicts of Interest:
- Remdesivir is an intravenous drug used in hospitals. It requires a treatment cycle of 5-10 days. A five-day course of treatment per patient costs around $2340 for government programs and $3,120 for private insurance. This is outrageous considering that Fauci gave Gilead Sciences $30 million of taxpayers’ money for their clinical trials in 2020. Not only that but remdesivir costs Gilead about $10 per dose.
- Hospitals receive a 20% “boost” bonus payment from Medicare on the entire bill for using remdesivir on patients – just like they receive monetary incentives for putting people on ventilators. In fact, hospitals have made more money in the past couple of years than pre-Covid while the health of Americans and life expectancy has declined.
- Gilead, the manufacturer of remdesivir, also manufactured AZT – the toxic drug that Fauci pushed to approve for the treatment of AIDS.
- Remdesivir was developed by Dr. Ralph Baric’s lab at UNC Chapel Hill. This is the same individual that Fauci funded to run gain-of-function research on coronaviruses during the moratorium. This guy has been collaborating for decades with scientists at the Wuhan Institute of Virology, in China.
- On January 2020 (before the pandemic was declared), the Wuhan Institute of Virology along with the Military Medicine Institute of the People’s Liberation Army of China sought a patent on remdesivir.
- US Army Medical Research Institute of Infectious Diseases – USAMRIID, the CDC, and Fauci hold patents for remdesevir, which means that they receive royalties for it.
- Gilead receives funding from the Gates Foundation and has deep financial entanglements with Fauci.
After realizing how much money is being made and identifying all the parties involved in the development and distribution of remdesivir, you understand why it was the first approved drug to treat COVID-19. Despite remdesivir’s toxicity, its poor effectiveness, and the manipulated results full of poor laboratory practices from Fauci’s funded studies, this is one of the official COVID-19 treatment protocols. No surprise there since financial entanglements between pharmaceutical companies, the CDC, FDA, and Fauci have become commonplace. Remdesivir is another factor contributing to the increased mortality rates post-2020.
To a Fitter Healthier You,
The Fitness Wellness Mentor